Werum IT Solutions has released the latest version of PAS-X Manufacturing Execution System (MES) for the pharmaceutical and biopharmaceutical industries. The new PAS-X 3.1.8 features several improvements for efficient manufacturing based on the latest technology standards. PAS-X 3.1.8 not only facilitates regulatory compliance, but also supports drug makers to ensure high quality in their manufacturing processes.
 |
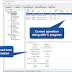
New dialog for material reconciliation
|
Ensuring manufacturing quality
The latest trends in pharmaceutical manufacturing have been considered and reflected in the new PAS-X. “PAS-X 3.1.8 is future-ready,” says Robert Welter, Senior Head of PAS-X Product Management at Werum IT Solutions. “Our user forum PAS-X For Us actively participated in advancing PAS-X 3.1.8.”
The latest trends in pharmaceutical manufacturing have been considered and reflected in the new PAS-X. “PAS-X 3.1.8 is future-ready,” says Robert Welter, Senior Head of PAS-X Product Management at Werum IT Solutions. “Our user forum PAS-X For Us actively participated in advancing PAS-X 3.1.8.”
“The use of PAS-X as an MES ensures data integrity and significantly reduces possible data integrity related issues,” adds Carsten Bierans, Senior Head of Quality Management at Werum. “Together with the PAS-X For Us working group the MES-related data integrity positions of the pharma & biotech MES user community are defined.”
Werum continues its usability initiative with a particular focus on quality in shop floor operations. With PAS-X 3.1.8, the most important material-related functions can now be accessed more easily and have been given an optimized user interface. Werum also introduced a dedicated dialog for material reconciliation with built-in best practices. The new automated material flow overview allows the operator to control the quantities that have been consumed and processed.
Improved update capabilities and infrastructure improvements
PAS-X customers will benefit from the significantly reduced downtime needed to update the software version. A short downtime is crucial for modern bio-pharmaceutical manufacturing processes. “We reduced the downtime by more than 50%,” says Marcel Ecks, Head of PAS-X Infrastructure & Development Tools at Werum. “We have achieved this high reduction rate not only through technical improvements, but through a holistic approach involving both the process itself and the documentation so that also the qualification efforts could be reduced.”
PAS-X customers will benefit from the significantly reduced downtime needed to update the software version. A short downtime is crucial for modern bio-pharmaceutical manufacturing processes. “We reduced the downtime by more than 50%,” says Marcel Ecks, Head of PAS-X Infrastructure & Development Tools at Werum. “We have achieved this high reduction rate not only through technical improvements, but through a holistic approach involving both the process itself and the documentation so that also the qualification efforts could be reduced.”
PAS-X 3.1.8 allows a cumulative one-step MES product upgrade and data migration. It runs with the latest platform versions such as Oracle 12. Support of the new OSIsoft PI Event Frames simplifies integration of shop-floor systems.
Seamless integration of latest PAS-X solutions
PAS-X 3.1.8 allows an even smarter integration of Werum’s proven PAS-X solutions such as Track & Trace, KPI/OEE and the new Evaluations Package. The new PAS-X Evaluations Package helps pharma manufacturers unlock the full potential of shop floor data. In PAS-X, all production-related data and data of the interfaced IT systems converge. Supported by the integrated and web-based PAS-X Evaluations Package, this combined information can be used to make production assessments and develop strategies for process optimization.
PAS-X 3.1.8 allows an even smarter integration of Werum’s proven PAS-X solutions such as Track & Trace, KPI/OEE and the new Evaluations Package. The new PAS-X Evaluations Package helps pharma manufacturers unlock the full potential of shop floor data. In PAS-X, all production-related data and data of the interfaced IT systems converge. Supported by the integrated and web-based PAS-X Evaluations Package, this combined information can be used to make production assessments and develop strategies for process optimization.
Simply the best: market-leading MES with all PAS-X 3.1 features
“With the latest PAS-X release, pharma manufacturers benefit from a comprehensive compilation of innovations, functionalities and usability improvements that Werum introduced throughout the PAS-X 3.1 family,” says Arndt Erdtmann, Senior Director PAS-X Development at Werum. For example, Werum customers benefit from automated execution features such as automated material flow and automated equipment identification, each of which improve user efficiency and reduce risk of human error. Data integrity is supported by alarm & events, directly retrieving GMP-relevant exceptions also from level 2 systems and enabling QM to significantly accelerate the release process of Batch Record Reports through review by exception. Further acceleration can be achieved by auto-closure functions, which unburden QM from checking batches without any exceptions and critical parameters at all, as manual release is no longer required.
PAS-X 3.1.8 contains the experiences and best practices of decades of successful MES projects in all areas of the pharma and biotech industries. Several Werum customers have already decided to incorporate PAS-X 3.1.8. Thorsten Grundmeier, Senior PAS-X Consultant at Werum, says: “With PAS-X 3.1.8, the most sophisticated and pharma-dedicated MES in terms of functionality and usability now becomes available on the market for customers in any region of the globe and from small- to large-scale production. It is simply the best solution to fully utilize the key benefits of MES for pharma and biotech manufacturers: compliance, quality and performance.”
@Werum #PAuto #Pharma

















No comments:
Post a Comment